Contents
State and Explain Coulomb’s Law
History of Coulomb’s law:
Coulomb’s law is named after Charles-Augustin de Coulomb, a French physicist who formulated the principle in the 18th century. Here’s a brief overview of the historical context and development of Coulomb’s law:
- Before Coulomb’s work, there was already an understanding of electric charge and basic electrostatic phenomena. Scientists like William Gilbert and Benjamin Franklin had conducted experiments and made observations regarding the behavior of charged objects. Franklin, in particular, proposed the concept of positive and negative charges, as well as the idea that like charges repel each other while opposite charges attract.
- Coulomb’s Experimentation: Charles-Augustin de Coulomb conducted groundbreaking experiments in the 1770s and 1780s to systematically study the forces between charged objects. His experiments involved suspending charged objects from thin strings and measuring the angle of deflection due to the electrostatic force between them. Coulomb’s experiments were meticulous and precise, laying the foundation for quantitative measurements in electricity.
- Formulation of Coulomb’s Law: Based on his experimental findings, Coulomb formulated his law, which quantified the relationship between the force, charges, and distance between them. He published his results in 1785 in a paper titled “Premier Mémoire sur l’Électricité et le Magnétisme” (First Memoir on Electricity and Magnetism).
- Legacy and Impact: Coulomb’s law revolutionized the study of electricity and magnetism. It provided a precise mathematical description of electrostatic forces, enabling scientists and engineers to develop new technologies and applications based on the principles of electromagnetism. Coulomb’s law laid the groundwork for the later development of Maxwell’s equations, which unified the theories of electricity and magnetism into electromagnetism.
What is Coulomb’s LAW:
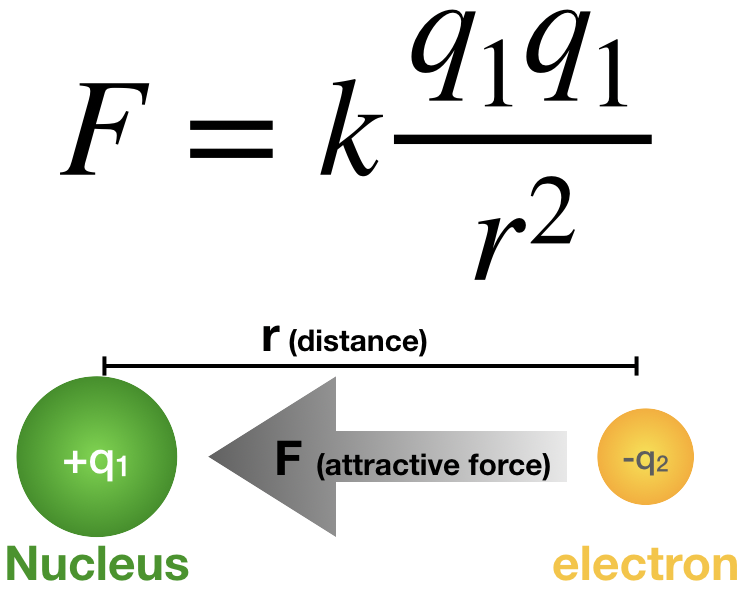
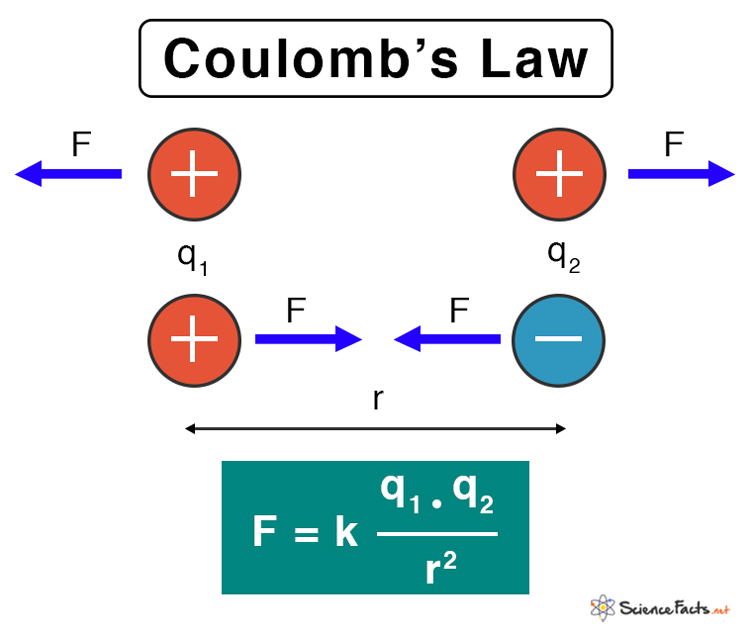
Coulomb’s law is a fundamental principle in physics that describes the electrostatic interaction between charged particles. “It states that the force ( F ) between two point charges is directly proportional to the product of their magnitudes and inversely proportional to the square of the distance between them.”
Mathematically, Coulomb’s law can be expressed as:
F=k⋅q1⋅q2/(r)2
Where:
- F is the magnitude of the electrostatic force between the two charges,
- 𝑞1q1 and 𝑞2q2 are the magnitudes of the two charges,
- 𝑟r is the distance between the charges,
- 𝑘k is Coulomb’s constant, a proportionality constant determined by the properties of the medium between the charges.
Description:
Certainly! Coulomb’s law describes the relationship between electric charges and the force they exert on each other. Let’s break down the components of the law and its significance:
- Force between charges: Coulomb’s law tells us how much force two charged particles exert on each other. If the charges are of opposite signs (one positive and one negative), they attract each other. If the charges are of the same sign (both positive or both negative), they repel each other.
- Magnitude of the force: The force 𝐹F between two charges is directly proportional to the product of their magnitudes (𝑞1q1 and 𝑞2q2). In other words, the larger the charges, the greater the force they exert on each other. If one charge is twice as large as the other, the force between them will be twice as strong, assuming all other factors remain constant.
- Inverse square law: The force between charges decreases with the square of the distance between them (𝑟r). This means that as the distance between two charges increases, the force they exert on each other decreases rapidly. If you double the distance between two charges, the force decreases by a factor of four. This inverse square relationship reflects how the electric field spreads out in three-dimensional space from a point charge.
- Coulomb’s constant (𝑘k): This constant is a proportionality factor that depends on the properties of the medium between the charges. In a vacuum, 𝑘k takes on a specific value (approximately 8.9875×109 N⋅m2/C28.9875×109N⋅m2/C2). In other materials, such as air or water, the value of 𝑘k may change slightly due to the material’s ability to conduct or insulate electric charge.
The direction of the force is along the line joining the two charges and is attractive if the charges have opposite signs (one positive and one negative), and repulsive if the charges have the same sign (both positive or both negative).
Coulomb’s law applies to point charges, which are charges that are concentrated at a single point in space and have no spatial extent. It is essential in understanding the behavior of electric fields, which are created by charges and influence the motion of other charges in their vicinity. This law plays a crucial role in various fields of physics, including electromagnetism, electronics, and electrostatics.

